Consulting for ISO 13485
MEDICAL DEVICES QUALITY MANAGEMENT
When it comes to medical devcies, ensuring that you continuously improve the quality of your organisation, products and services to ensure you meet your customers’ and regulatory expectations is critical.
Start your journey to ISO13485!

Start your journey to ISO13485 !
The benefits of working with
Many Caps Consulting
Keeping It Simple
Our focus is to ensure your systems are simple to understand and use.
No Hidden Costs
We'll tell you up front the maximum you will pay for our support.
We Are Your ISO Coaches
We work as your coach, we help you learn 13485 and develop your systems.
The benefits of working with Many Caps Consulting
100% Success Rate
100% of our clients achieve their ISO13485 Certification with us.
Real World Experience
All of our consultants have worked in the real world with ISO13485.
Stress Free Certification
We work with you to make sure that the whole process is stress free.
Why certify to ISO13485 for Medical Devices Quality Management?
The Benefits
of an ISO13485 Certification
Here are just a few of the benefits of certifying to ISO13485 for Medical Devices Quality Management Systems for your organisation:
It's the International Standard for Quality
Achieving ISO13485 for Medical Devices Quality Management Systems reflects your organisations desire to set and maintain great quality management systems that everyone can count on. It means it is going to be recognised by your customers, suppliers, regulators and your competitors world wide. Plus suport you in meeting the requirements of the FDA in the US or the European Medical Devices Directives
Improved Performance
An ISO13485 Medical Device Quality Management System ensures that you have the systems in place that you need to effectively run the organisation and that everyone knows about them, that way you have one way of doing things which ultimate cuts wasted costs and rework.
Employee Engagement
Your ISO13845 System is created and owned by its users, your employees. That means they get input into your business processes, setting and improving your systems. They are actively engaged to help continuously improve the system that they use in order to make it better for everyone.
Customer Satisfaction
ISO13485 has a requirement to seek feedback from your customers on how you are doing and via post market surveillance to look for wider trends and issues. These feedback loops allow you to look at how you can improve your business from the point of view of the customer. It helps you identify the areas you need to continuously improve if you are going to keep your customers satisfied.
Independent Certification
Knowing that an external auditor has assessed what you do and confirmed it meets the high standards of the ISO13485 certification gives your employees, suppliers and customers confidence in your organisation.
Expert ISO13485 Support With Practical Application
Achieve ISO Certification
Simply, Quickly and Cost Effectively
Achieving your ISO13485 certification may seem like a daunting task. Trying to understand what each of the clauses mean and what the requirements are can sometimes seem harder than it should be. That is where our expertise in ISO Management Systems can help.
Experts with real experience
We specialise in helping Kiwi businesses not just achieve their ISO13485 certifications but maintain it and ensure it adds value to their businesses rather than being one more thing to do. We have been working in complianxce environments for over 20 years in the real world. We have seen it change from a tick the box and regimented structure to the current ISO13485 version which is focused on risk based thinking and continuous improvement.
Teaching you to fish
Our ISO consultants will help you understand what the requirements of the ISO standards are in plain English. They will help you develop the required documents, processes and systems that will give you the tools to run an ISO13485 Medical Devices Quality Management System that genuinely adds value to your business.
Start your journey to ISO13485 !
Practical Focus
We focus on taking a practical approach to implementation and keeping things simple. Success for us is that when we step back you know your systems and how to run them, that is why our expert ISO13485 consultants focus on coaching your team rather than implementing a cookie cutter solution.
We work at your pace
We won't hand you a prewritten ISO13485 Medical Devices Quality Management System Manual. We want to help you create your manual for your company so it sounds like you and uses your words. That can take a little time, that's Ok, you should take the time to think about how you actually do things and involve your team. We'll work with you at your pace, not ours.
Check our references
What do people say
about Many Caps Consulting?
Don't just take our word about working with us, here is the feedback from some of our clients.
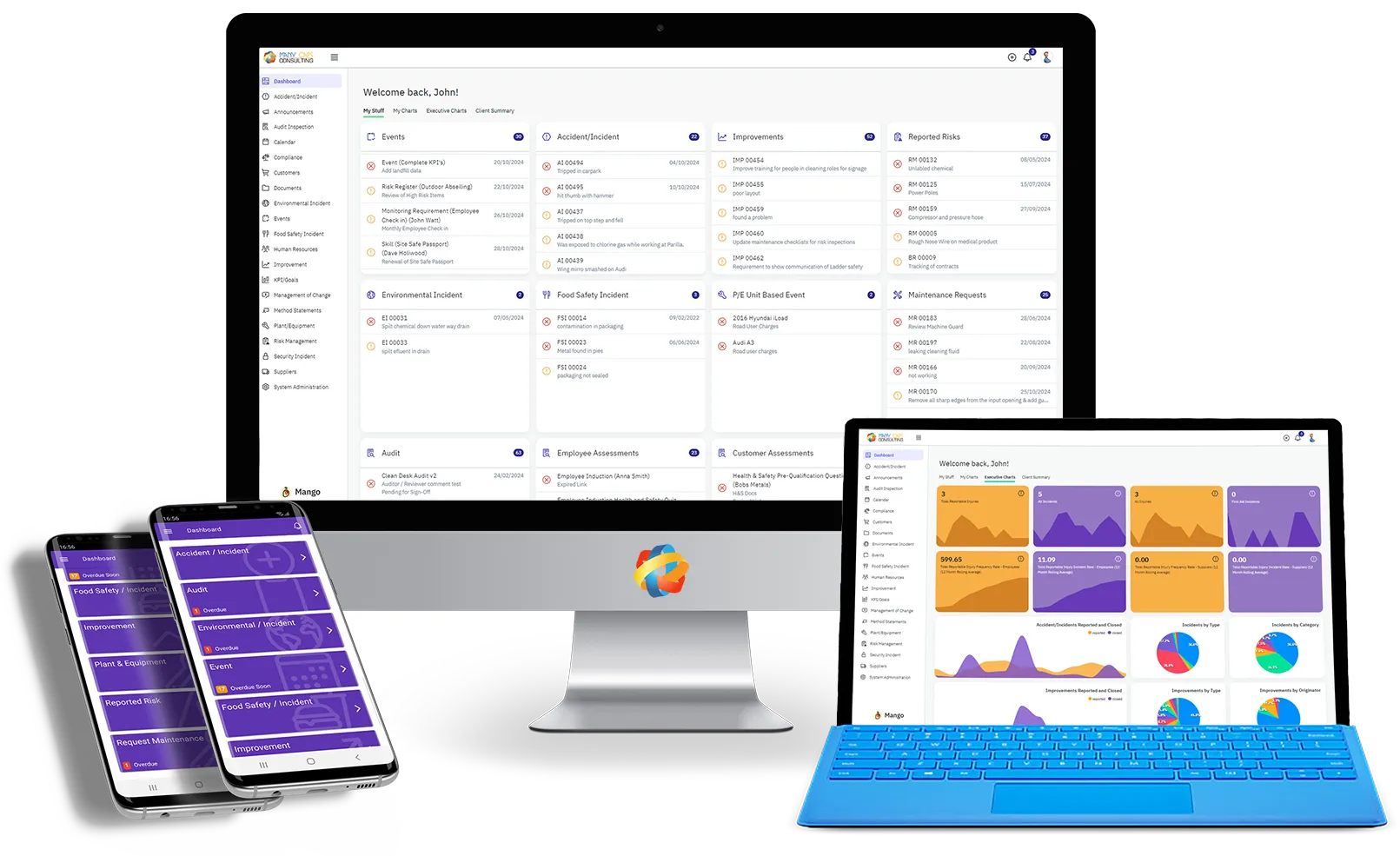
We Recommend Mango

Mango QHSE is a fully integrated SAAS cloud-based compliance system which dramatically simplifies, integrates and accelerates the creation and use of your ISO Management System.
With dedicated modules for Document Control, Customer & Supplier Management, Improvement & Non-conformances, Risk Management, Auditing and Plant & Equipment Maintenance this is a single solution that can be used across all ISO Management System requirements.
Make time for a chat
Book your consultation
let's talk about achieving 13485
If you are considering getting ISO13485 for Medical Devices Quality Management Systems it's never too soon to talk with us about what is involved. We'll guide you through the entire process and make it as simple as possible, no commitments, no hassle, just a chat.





